BACKGROUND
In Sickle Cell Disease (SCD), hypoxia favors deoxy-HbS formation, sickle hemoglobin (Hb) polymerization, and red blood cell (RBC) sickling. The hypobaric hypoxia of high altitude (such as plane flights and high-altitude vacation destinations) may uncover occult hypoxia in people with SCD, trigger vaso-occlusive episodes, and/or exacerbate cardiopulmonary complications. There are few published data on high-altitude hypoxia in people with SCD. High altitude simulation testing (HAST) recapitulates the hypoxia of high altitude and can be used to assess the need for prophylactic supplemental oxygen prior to trips. At our institution, due to anecdotal reports of symptom exacerbation following air travel, we complete pretravel HAST for people with sickle cell disease, and then prescribe portable oxygen therapy.
OBJECTIVE
This retrospective cross-sectional study aimed to assess the prevalence of high-altitude hypoxia in those adults with SCD who underwent pretravel evaluation from 2019 to 2023. Additionally, we examined the clinical characteristics of those people found to have high-altitude hypoxia.
METHODS
After getting IRB approval, we retrospectively reviewed the medical records of patients with SCD who had undergone HAST as part of pretravel evaluation in 2019 through 2023.
HAST was performed with the use of a Hans Rudolph two-way non-rebreathing valve system, and oxygen saturation was monitored with a forehead oximeter. Compressed Air (10 liters per minute) and Nitrogen (8 liters per minute) were blended to an oxygen level of 15%, simulating an altitude of 8,000 feet for 20 minutes. High-altitude hypoxia is defined as persistent SpO 2 ≤88% at 15%FiO 2, requiring supplemental oxygen. Demographics, SCD-related medical history, clinical labs and imaging were collected near the time of testing.
We assessed the prevalence of high-altitude hypoxia in our patient population over the time period. We described the characteristics of those tested. Using parametric and non-parametric statistical tests, we evaluated associations between time to hypoxia (in minutes).
RESULTS
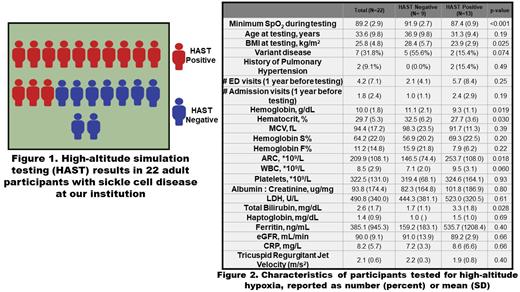
22 adult patients underwent HAST, and 13 (59%) were positive for high-altitude hypoxia (Figure 1). The time to hypoxia ranged from 1-17 minutes with a mean of 5.3 minutes with a minimum SpO 2, in those that tested positive, ranging from 86-89%. However, hypoxic patients were immediately treated with supplemental oxygen during testing, so a true nadir cannot be determined.
Compared to those who tested negative, the participants that tested positive had lower BMI and lower mean Hb and hematocrit levels. Their mean absolute reticulocyte counts (ARC) and mean total bilirubin were higher than those who tested negative, and their mean white blood counts (WBC) trended higher. We did not observe significant difference in HAST results by SCD genotype, hydroxyurea use and opioid use. Other patient characteristics are described (Figure 2). We found the strongest inverse correlations between time to hypoxia and WBC (r= -0.73, p= 0.004) and ARC (r= -0.69, p=0.010).
CONCLUSIONS
Adults with sickle cell disease are at risk for occult hypoxia during travel due to hypobaric hypoxia associated with altitude. In our experience, screening patients with SCD prior to travel, we found HAST-positive hypoxia in 59%, for whom we prescribed supplemental oxygen during travel. Our observations suggested that people with more ‘active’ SCD (lower Hgb and higher ARC) were more likely to have high-altitude hypoxia and tended to declare their hypoxia earlier in the testing period. Our study is limited by sample size, retrospective design, and a lack of patient reported outcomes about altitude associated symptoms or complications. Additionally, there may be a selection bias towards healthier people with SCD, i.e., those who are well enough to travel. However, given the pathophysiology of SCD, our results suggest that providers should be proactive in inquiring about travel plans and assessing the risk for hypoxia. Furthermore, the development of a brief screening tool in this setting may be warranted.
Disclosures
LeVarge:United Therapeutics: Membership on an entity's Board of Directors or advisory committees. Little:Pfizer: Consultancy; Novo Nordisk: Consultancy; NHLBI: Honoraria; USC: Research Funding; bluebird bio: Consultancy; Biochip Labs: Patents & Royalties: Make no profit; FORMA: Other: Adjudication committee for Hibiscus study; Hemex: Patents & Royalties: Make no profit; GBT: Research Funding; NASCC: Research Funding; American Society of Hematology: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal